Valence electrons and chemical bonding were always my favorite parts of chemistry. However, it was only recently that I began to view them in the context of origins. This article will discuss valence electrons, how they are used for chemical bonding, and how this special type of bonding fits into the origins debate.
As a starting point, every element has three components to it. These elements are protons, neutrons, and electrons. Protons come with positive charges, neutrons have no charge and electrons come with a negative charge. A positive charge is represented in chemistry as a plus sign, while a negative charge is represented as a negative sign. Protons and neutrons make up the core of the element, while electrons whiz around the outside in what is called electron shells. Elements do not all have the same number of electrons. The number of electrons in an element is equal to the number of protons unless the element acquires a charge by losing or gaining an electron. However for elements to bind to one another, they each must have the correct number of electrons in the outermost electron cells, called the valence shells. Most valence shells require eight electrons to be full, with some exceptions, notably hydrogen which requires two. Keep hydrogen’s electron requirements in mind. They will be important later.
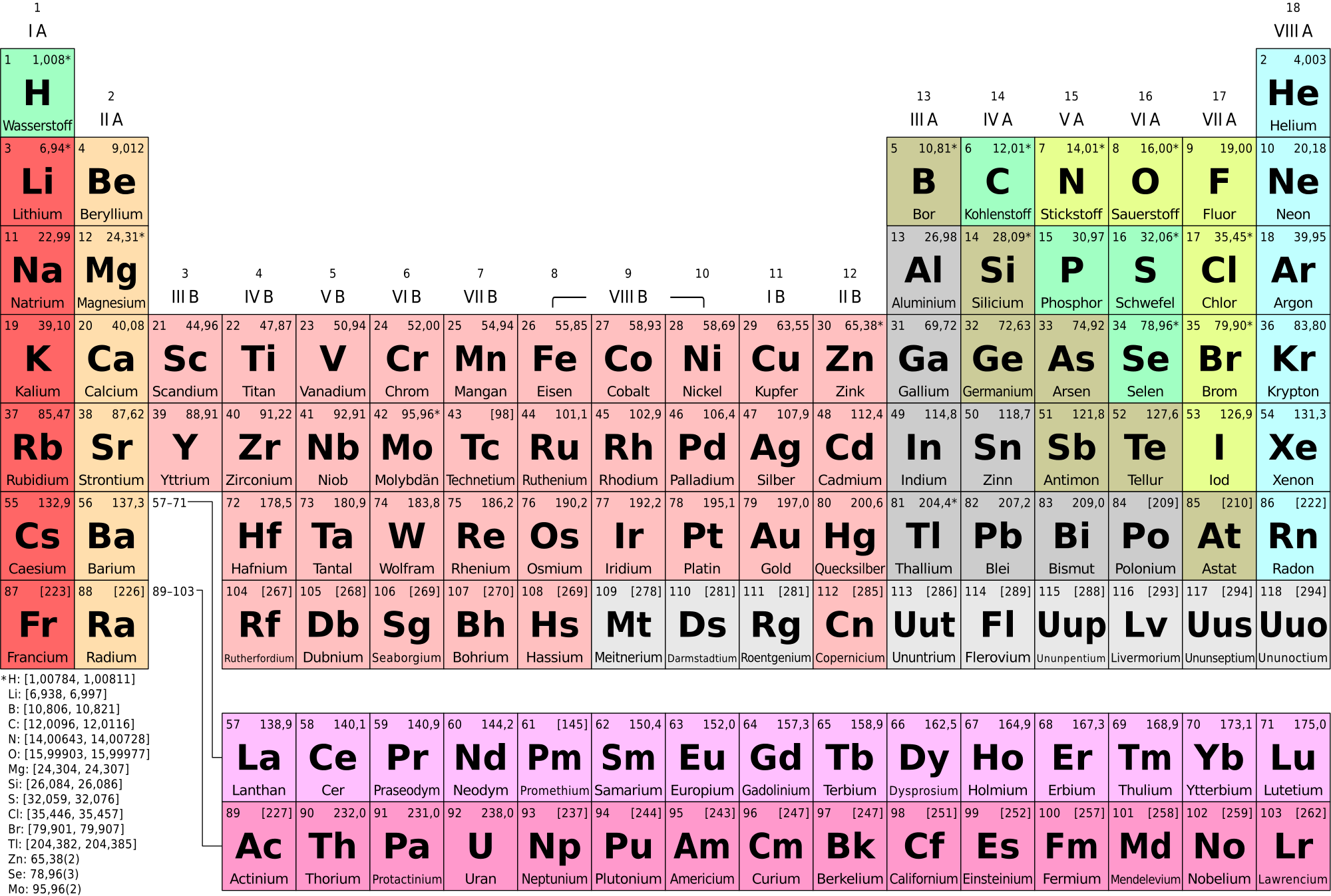
Determining how many electrons are in the valence shell of a given element is relatively easy if a periodic table is before you. With a few exceptions, reading across the periodic table from left to right will inform you how many electrons are in each valence shell. This does not work for transitional metals in the middle of the table, but for the outer parts of the table, this works quite well. The number of valence electrons for a given element is sometimes represented using the abbreviations for the element, and up to eight dots on the outside, representing the electrons. Such a configuration for oxygen is shown below. Because these electrons are in the valence shells, they are furthest from the nucleus, and thus the most easily removed from the element. Elements with eight valence electrons are considered stable, and largely do not react with other elements or form bonds. This is why elements bond together. By sharing electrons, each can reach a stability un-achievable on its own. The kind of bond formed by sharing electrons is called a covalent bond. However, sometimes, particularly when metals are involved, an ionic bond will form instead, resulting in one element gaining an electron, and another losing one.

Covalent bonds are formed by electrons being shared between elements so that each element has eight electrons in its valence shell. Covalent bonding can take the form of single, double, or triple bonds, depending on how many electrons are involved. A single bond involves sharing one electron, a triple bond three. There are two types of covalent bonds, pi bonds, and sigma bonds. Which variant forms is dependent upon the way that the electrons involve orbit the nucleus of the elements. Sigma bonds comprise most single bonds and are stronger than pi bonds. Double bonds typically consist of one of each type, while triple bonds contain one sigma and two pi bonds. Because the elements that covalently bond do not give up an electron in the bond, neither gains a charge and thus the attraction between them is limited. This allows the elements in the bond to move around freely, meaning that most covalent bonds are found in liquids and gases. Probably the most well known covalent bond is found in water. Oxygen, as depicted above, has six valence electrons, meaning it needs two to be full. Each hydrogen only has one valence electron, but, as noted above, needs only one more to have a full valence shell. Thus when two hydrogens share electrons with an oxygen, each element has a full valence shell and is satisfied. The resulting molecule is the water molecule and drawn as follows. Notice that four electrons have been replaced with two lines. Each line represents two electrons. All three elements are satisfied. Each Hydrogen has two electrons and Oxygen has eight; four shared and four all to itself.

Now that I’ve established a basic background, let us examine covalent bonding using valence electrons in the light of origins. Obviously, for covalent bonds to form, elements must exist. Chemical evolution is not something evolutionists talk about very much, likely because it is both more difficult for them and because chemicals are not cute and cuddly like some animals. However, for evolution to be true, chemical evolution must have occurred. Natural selection cannot be the mechanism here, as chemicals do not have DNA for it to select from. Therefore evolutionists must devise a new mechanism. They have failed utterly on this front. There is no coherent theory to explain the origin of chemicals. And even if there were, it would be logical to assume that chemicals would evolve to be satisfied, ie have a full valence shell. However, since this would prevent covalent bonding, most liquids and gases would be impossible to form, including water. Further, even supposing chemicals evolved to not have complete valence shells, why would they choose to share an electron over simply taking one? Why are all bonds not ionic? Evolution would predict that each element would simply do its best to get as many electrons as it could, regardless of where it got them. Covalent bonding is the opposite of what evolution would predict. It makes much more sense to say that God designed chemicals to have incomplete valence shells so that they would be able to share electrons and undergo covalent bonds, enabling gases and liquids to exist.
Do you know what’s going to happen when you die? Are you completely sure? If you aren’t, please read this or listen to this. You can know where you will spend eternity. If you have questions, please feel free to contact us, we’d love to talk to you.


1 Comment